New Technology Spotlight…
Bath Ratio and Temperature Control
Enhancement in the Potroom
By Dr. Marc Dupuis, GeniSim Inc.
ath chemistry and
order to determine the bath ra-
cell operating tem-
tio.1 When a cryolitic melt is
perature in Hall-
cooled from a liquid state to
B
Héroult aluminum
a solid state, it goes through
reduction cells must be con-
several phase transformations.
trolled to achieve optimal
Each transformation occurs in
current and energy efficiency.
a specific temperature region,
The conventional way to con-
therefore the bath chemistry
trol the bath ratio and temper-
ratio is proportional to the
ature is to regularly take bath
amount of heat release.
samples for chemistry analysis
Based on this method, the
and measure the bath tempera-
probe tip contains two type K
ture. These measurements are
thermocouples
(Figures
2-3)3
generally performed separate-
that allow these unique cool-
ly. Moreover, bath samples
ing characteristics to be mea-
have to be sent to a laboratory
sured and the bath ratio to be
for analysis, with results avail-
determined. The thermocouple
able in as much as 24 hours
on the left records the cooling
later. Due to the delay in get-
rate of the bath sample, while
ting the bath sample analysis
the thermocouple on the right
results, control decisions have
records the cooling rate of the
to be made primarily relying
metallic mass of the probe.
on old and out of sync infor-
With this probe, the ratio re-
mation. This leads to an un-
Figure 1. STARprobe kit with mobile station and monitoring system
sult can be known after only a
(left) and portable stand showing the probe tip, lance, and head ready
steady feedback control loop,
few steps—insert probe tip into
for measurement (right).3
where the cell is continuously
molten bath to equilibrate with
under or over shooting the tar-
bath temperature in the pot cell,
geted optimum conditions, which causes
exists a high magnetic field, high ambi-
remove probe tip from bath and allow it
sub-optimal cell performance in terms
ent temperature, and a highly dusty en-
to cool, and STARprobe analyzes the
of both current and energy efficiency.1,2
vironment. This system is mounted on
cooling curve and records the results.
To address this problem, over the last
a cart to make it a mobile station with
10-15 years, Alcoa has worked to de-
spare probe tips and a battery backup to
velop a new device to measure almost
increase the PC tablet autonomy.
instantly the bath chemistry (or excess
Since the probe tip is reusable, a com-
AlF3) and temperature. The Superheat,
plete measurement cycle requires only a
Temperature, Alumina concentration,
few steps and can take just under 4 min-
and Ratio (STAR) analysis system,3,4
utes to complete. Furthermore, a single
known as STARprobe™, provides real-
PC tablet can process data from two
time results that allow potroom op-
probe heads simultaneously, allowing
erators to make chemical alumina and
the operator to measure two pot cells in
power adjustments for optimal cell per-
parallel. Considering that a probe tip is
formance. To date, it has been success-
able to take around 100 measurements,
fully deployed in eight Alcoa smelters.
in this way, a trained operator can rou-
tinely measure 64 cells in 4 hours with
Figure 2. Reusable probe tip.
System Overview
an average of 3 minutes and 45 seconds
per measurement.
The STARprobe system (or kit) is
Once the data is available, the STAR-
a portable device that takes real-time
probe system makes use of the well-
measurements of bath properties and
known Differential Thermal Analysis
consists of four major components (Fig-
(DTA) method,5 the results of which are
ure 1): a reusable probe tip (Figure 2);
displayed on the tablet screen (Figure
a portable probe stand (lance) that can
3), stored in a file, and transmitted to the
fit in various smelter operations for di-
level 2 control system in the smelter, us-
rect measurement of the bath; electron-
ing an Alcoa QLC cell controller system.
ics to acquire data and perform analy-
sis, which is wirelessly transmitted to
DTA Method
a computer server; and the STARprobe
computer program that integrates with
The STARprobe concept is fairly
a PC tablet.1 All electronic components
simple and uses the DTA measure-
comply with stringent requirements for
ment method, which analyses cooling
Figure 3. Schematic of the STARprobe tip
operation in the potroom, where there
characteristics of the cryolite melt in
with two type-K thermocouples.
20
LIGHT METAL AGE, FEBRUARY 2013
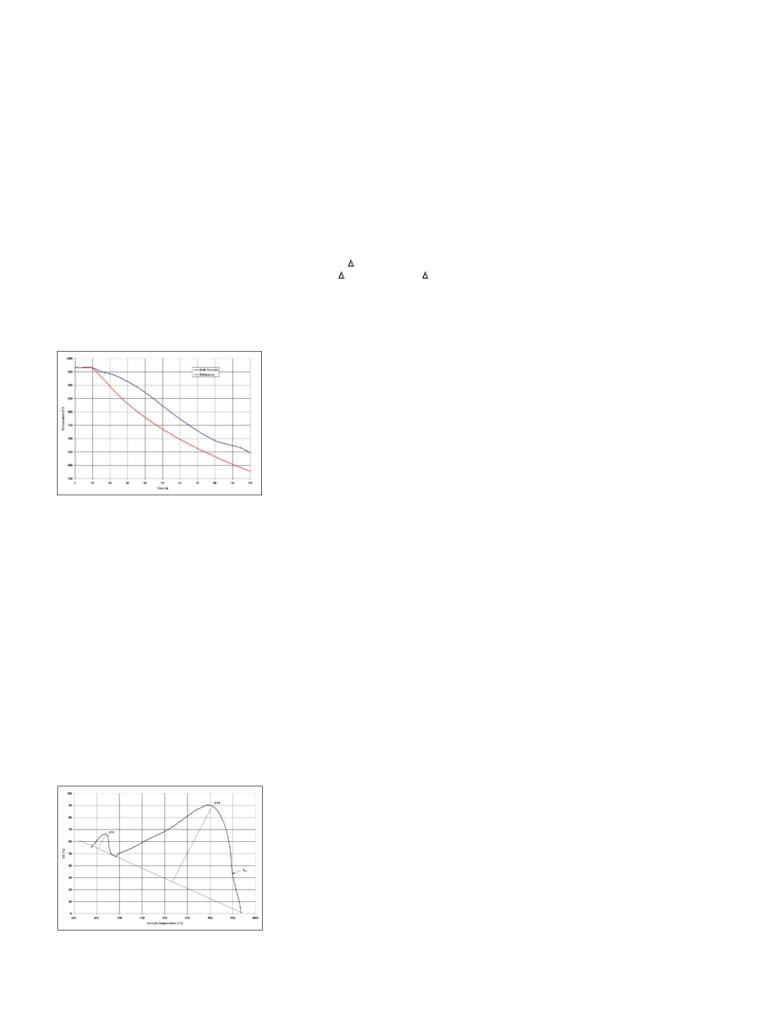
Figure 4 presents such a pair of DTA
that could identify the bath composition
one of the Alcoa smelters now using the
curves obtained from a STARprobe
from the shape of each curve measured.
STARprobe to control its bath ratio and
measurement. In this case, the cooling
The high temperature maximum is due
temperature, reported that the technology
rate of the bath sample is slower than
to the solidification of the cryolite,
has proven to be very accurate for ratio
the metallic mass of the probe for two
while the low temperature maximum is
(excess AlF3) measurements and able
reasons. The first and less significant
mainly due to the solidification of the
to replace traditional bath sampling and
reason is because of the difference of
excess AlF3. The more AlF3 in the col-
XRD laboratory analysis.8 Alumar also
thermal diffusivity between the bath
lected bath sample, the less intense the
reported the advantages of much faster
sample (liquid and solid) and the me-
high temperature peak will be and the
response time and consequent reaction on
tallic mass of the probe, hence the ini-
more intense the low temperature peak.
chemical additions, faster measurement
tial separation of the two curves be-
Mathematically, this means that the
of bath superheat and alumina concentra-
tween 10 and 18 seconds. Second, at
sample bath ratio (or excess AlF3 con-
tion, ability to transfer data to pot con-
the bath sample liquidus temperature,
centration) correlates with the ratio of
trollers that are used to modify process
cryolite starts to solidify, which slows
intensity of the two peaks as displayed
control regarding thermal control, and
the bath sample cooling rate down
in the following equation:1
ability to reject bad results and request
even further. At the cryolite-alumina
a recheck measurement immediately.
%xs AlF3 = f1[ΔHAlF3/Na5Al3F14 /
phase diagram bath eutectic tempera-
Test groups are pointing to a big poten-
(ΔHAlF3/Na5Al3F14+ ΔHNa3AlF6)]
ture, the alumina starts to solidify as
tial in terms of voltage reduction as well
well. Finally, at a much lower temper-
= f2[S2/(S1+S2)]
as reduced aluminum fluoride additions.
ature (at the cryolite-AlF3 phase bath
Also, test groups have shown good levels
= f3[DT2/(DT1+DT2]
eutectic temperature), the excess AlF3
of superheat with a decreasing trend after
finally solidifies.
The method used by the correlations
starting the STARprobe measurements.
algorithm to calculate DT1 and DT2
is not described in Alcoa’s published
Conclusion
research, as it remains a trade secret.
However, even without the latent heat
The new STARprobe bath properties
released during the solidification of the
measurement device is offering smelters
bath sample, the reference temperature
a significant potential to improve pro-
drifts apart from the sample temperature
cess efficiency. While Alcoa is not cur-
(Figure 4 illustrates a possible definition
rently licensing its QLC or its active pot
of DT1 and DT2 by the author that may
control logic, the company has selected
or may not be close to Alcoa’s correla-
STAS as worldwide distributor for its
tion algorithm research). For sure, Al-
revolutionary STARprobe technology.
coa’s correlation algorithm is fast and
Figure 4. Recorded cooling rate of bath sam-
the calculated results are comparable to
com/en/starprobe/html.
ple and the metallic mass of the probe, which
XRD analysis and have been indepen-
act as reference temperatures in the DTA.
dently verified on many occasions in
References
demonstrations preformed in smelters
The difference of temperature be-
around the world.6
1. Wang, Xiangwen, Bob Hosler, and
tween the two curves is computed and
Gary Tarcy,
“Alcoa STARprobeTM,”
presented on a second graph (Figure 5).
Process Control Improvements
Light Metals 2011, pp. 483-489.
In this case, the sample temperature is
Achieved by Alcoa
2. Dupuis, M., “Excess AlF3 concen-
selected as an X coordinate. The shape
tration in bath control logic,” National
of that curve is independent of the cool-
In parallel with the development of
Conference on Advancements in Alu-
ing rate, so the bath sample analysis re-
the STARprobe, Alcoa developed a new
minium Electrolysis, Indian Institute of
sults will not be affected by fluctuation
cell controller called QLC that takes full
Metals, Angul Chapter, 2006.
of the ambient conditions.1 In fact, the
advantage of its bath properties mea-
3. Hosler, Bob, Xiangwen Wang, Jay
shape of the curve depends only on two
surement technology. QLC automati-
Bruggeman, and Patrick O’Connor,
things, the design of the probe tip and
cally acquires the results of STARprobe
“Molten Cryolytic Bath Probe,” U.S.
the composition of the bath sample. This
measurements in real time and takes
Patent No. 2005/0069018 A1, 2005.
means that for a given probe tip design,
the measured cell superheat in consid-
4. Wang, Xiangwen, Bob Hosler,
the shape of the curve uniquely depends
eration in its new STARprobe-based
and Gary Tarcy, “Systems and Meth-
on the composition of the bath sample.
active pot control logic.1,7 The gains re-
ods Useful in Controlling Operation of
This is the reason Alcoa was able to
ported by Alcoa are 0.5% improved cur-
Metal Electrolysis Cells,” U.S. Patent
come up with a correlation algorithm
rent efficiency, 35 mV voltage savings,
No. 2007/0295615 A1, 2007.
5% AlF3 savings, a one time capital cost
5. Mackenzie, R.C., Differential Ther-
saving (X-ray equipment), labor savings
mal Analysis, Academic Press, London,
for sampling/analysis, and improved
1970.
understanding by operators, as well as
6. Dupuis, M., P. Bouchard, and J.-P.
a potential of 100-150 day potlife im-
Gagné, “Measuring bath properties us-
provement (still being tested).
ing the STARprobe™,” 19th Internation-
The potential for improvement for
al ICSOBA Symposium, 2012.
a given smelter depends on its current
7. Wang, X., G. Tarcy, E. Batista, and
level of process efficiency. For example
G. Wood, “Active pot control using Al-
the two cases of current efficiency (CE)
coa STARprobe™,” Light Metals 2011,
improvement reported by Alcoa were
pp. 491-496.
from about 94% moving up to about
8. Silva, Ari F., et al., “Implementa-
94.5%;7 clearly, a smelter already operat-
tion of STARprobe™ Measurements
Figure 5. Differential temperature curve and
one possible way to perform the DTA analy-
ing at 95.5% CE should not expect the
and Integrated Pot Control at Alumar,”
sis.
same level of improvement. Alumar,
ICSOBA Symposium, 2012.
LIGHT METAL AGE, FEBRUARY 2013
21